

The rate of a reaction is the number of successful collisions occurring between reactants within a certain amount of time. It is determined by the collision theory. This theory states that for a reaction to occur the particles must collide with sufficient energy and at the right orientation. Imagine putting a key into a lock either backwards or with barely any energy to turn the key – the door just wouldn’t be unlocked!
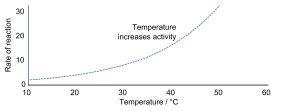
Increasing the temperature of a reaction increases the rate of reaction. The heat energy is converted into kinetic energy in the particles. More kinetic energy means that the frequency of collisions between particles increases because they’re moving around much faster. This increases the frequency of successful collisions.
(In Biology, enzymes in the body denature at high temperatures so in this case the rate of reaction wouldn’t increase as temperature increases past an optimum.)
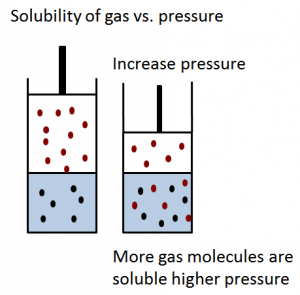
Increasing the pressure of a reaction involving gases increases the rate of reaction.
This is because it forces the gas particles to be closer together and are more likely to collide successfully. More successful collisions lead to a faster rate of reaction.
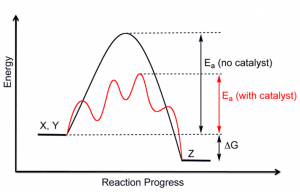
A catalyst provides an alternative route for the reaction to occur at a lower activation energy. This means that less energy is required for the reaction to occur. Therefore, the frequency of successful collisions increases, and the rate of reaction increases.
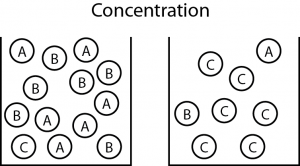
Increasing the concentration means there are more particles in a particular volume. If there’s more particles, then it increases the chances of successful collisions occurring. Increased successful collisions means greater reaction rates!
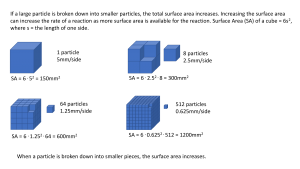
Increasing the surface area of the reactants increases the number of exposed particles that are available to react. As a consequence, the number of successful collisions increases and the reaction rate increases.
If you haven’t already noticed from all the repetition, the keywords or phrases in this topic are:
Reaction rates/Rate of Reaction
Frequency of successful collisions
Increase/decrease
Activation energy
Particles
Collide
Examiners are specifically going to be looking out for these keywords and phrases as it shows you understand the topic well – make sure you include them in your explanations!
Click here for a really good website for A Level Chemistry practise papers and topic homework.
Click here to watch a Youtube video by TED Education on Rates of Reaction.
Have a look at our blog post about revision techniques!
